There are many products marketed in the United States as gowns, other apparel, and gloves that offer a range of protection against potential health hazards. Gowns, other apparel, and gloves are regulated by FDA when they meet the definition of a device under section 201(h) of the FD&C Act.
Generally, gowns, other apparel, and gloves fall within this definition when they are intended for a medical purpose, including for use by health care professionals. Gowns, other apparel, and gloves that are not intended for a medical purpose, are not medical devices, as described in further detail below.
Gowns and other apparel are products intended to protect the wearer from the transfer of materials in the wearer’s environment. Gloves are products worn on a hand or finger to protect the wearer from the transfer of materials in the wearer’s environment.
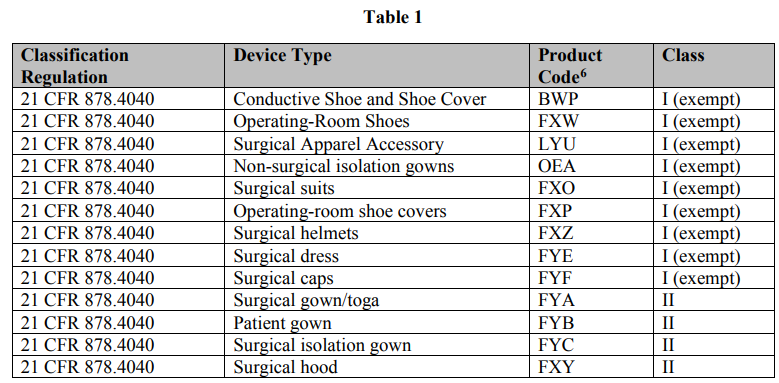
The classification regulation and associated product codes for FDA-regulated gowns and other apparel to which the policies in this guidance apply are listed in Table 1:
For the purposes of this guidance document:
Minimal or Low Barrier protection refers to:
- ANSI/AAMI PB70 Level 1 protection or equivalent; or
- ANSI/AAMI PB70 Level 2 protection or equivalent. Moderate or High Barrier protection refers to:
- ANSI/AAMI PB70 Level 3 protection or equivalent; or
- ANSI/AAMI PB70 Level 4 protection or equivalent.
Gowns and Other Apparel Not Intended for a Medical Purpose
Gowns and other apparel are devices when they meet the definition of a device set forth in section 201(h) of the FD&C Act. Under section 201(h) of the FD&C Act, these products are devices when they are intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease.
Other gowns and other apparel are marketed to the general public for general, non-medical purposes, such as use in construction and other industrial applications. Because they are not intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, when marketed for these non-medical applications, FDA device marketing authorization is not required, and all the other requirements of the FD&C Act do not apply to manufacturers, importers, and distributors of these products.
Gowns and other apparel are devices when they are intended for a medical purpose, such as prevention of infectious disease transmission (including uses related to COVID-19). Gowns and other apparel are not devices when they are intended for a non-medical purpose, such as for use in manufacturing or research and development. When evaluating whether these products are intended for a medical purpose, among other considerations, FDA will consider whether:
1) they are labeled or otherwise intended for use by a health care professional;
2) they are labeled or otherwise for use in a health care facility or environment; and
3) they include any drugs, biologics, or anti-microbial/anti-viral agents.
Non-surgical Gowns and Minimal-to-Low Barrier Protection Surgical Apparel
When evaluating whether a gown under 21 CFR 878.4040(b) is not a “surgical gown,” FDA will consider whether:
- it is labeled as a gown other than a surgical gown (e.g., isolation gown);
- it is not described in its labeling as a surgical gown; and
- it includes statements relating to barrier protection, and such statements are for only minimal or low barrier protection (e.g., ANSI/AAMI PB70 barrier protection Level 1 or 2).
Moderate-to-High Barrier Protection Surgical Gowns
When evaluating the classification of a gown under 21 CFR 878.4040(b), per FDA guidance, FDA will consider whether:
- it is labeled as such;
- it is described as such in its labeling;
- it has statements relating to moderate or high-level barrier protection; and/or
- it has statements that it is intended for use during sterile procedures.
In general, FDA recommends that health care providers follow current Centers for Disease Control and Prevention (CDC) guidance regarding personal protective equipment that should be used during the COVID-19 outbreak. Health care employers must also comply with standards of OSHA that require PPE to protect workers and that apply to infectious disease hazards. FDA recognizes the urgent need during the COVID-19 public health emergency for moderate-to-high barrier protection surgical gowns due to increased use and demand which has led to shortages in their availability. To ensure the availability of these types of surgical gowns during the COVID-19 public health emergency, FDA does not intend to object to the distribution and use of ANSI/AAMI PB70 Level 3 moderate-to-high barrier protection surgical gowns that do not comply with the following regulatory requirements, where such surgical gowns do not create an undue risk in light of the public health emergency: Prior submission of a premarket notification under section 510(k) of the FD&C Act and 21 CFR 807.81, Registration and Listing requirements in 21 CFR 807, and Unique Device Identification requirements in 21 CFR Part 830 and 21 CFR 801.20. FDA currently believes such devices would not create such an undue risk where:
- The product:
- Meets liquid barrier protection at Level 3 or higher, consistent with ANSI/AAMI PB70 for the critical zone areas;
- Meets the Class I or Class II flammability standard per 16 CFR Part 1610; and
- Has been demonstrated to be sterile if intended for use in surgical settings.
- The product includes labeling that accurately describes the product’s sterility status (sterile or non-sterile), including any sterilization method used, barrier protection as Level 3, flammability classification (Class I or Class II), and a list of the body contacting materials;
- The product includes labeling with general statements and makes recommendations that would sufficiently reduce the risk of use, for example, a general statement about devices that have not been cleared by FDA, recommendations against use when FDA-cleared surgical gowns are available, and recommendations against use of non-sterile products in surgical settings; and
- The product is not intended for any use that would create an undue risk in light of the public health emergency, for example, the labeling does not include uses for antimicrobial or antiviral protection; uses for infection prevention or reduction; or is labeled as having ANSI/AAMI PB70 Level 4 liquid barrier protection.
Full Guidance: https://www.fda.gov/media/136540/download

