There are many products marketed in the United States as gowns, other apparel, and gloves that offer a range of protection against potential health hazards. Gowns, other apparel, and gloves are regulated by FDA when they meet the definition of a device under section 201(h) of the FD&C Act.
Generally, gowns, other apparel, and gloves fall within this definition when they are intended for a medical purpose, including for use by health care professionals. Gowns, other apparel, and gloves that are not intended for a medical purpose, are not medical devices, as described in further detail below.

owns and other apparel are products intended to protect the wearer from the transfer of materials in the wearer’s environment. Gloves are products worn on a hand or finger to protect the wearer from the transfer of materials in the wearer’s environment.
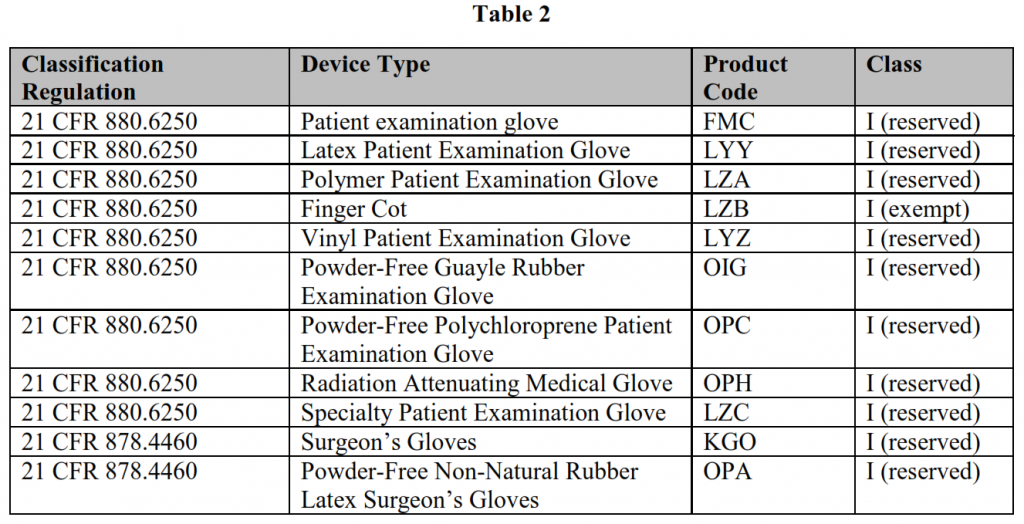
FDA-regulated gloves to which the policies in this guidance apply are listed in Table 2:
For the purposes of this guidance document:
Minimal or Low Barrier protection refers to:
- ANSI/AAMI PB70 Level 1 protection or equivalent; or
- ANSI/AAMI PB70 Level 2 protection or equivalent. Moderate or High Barrier protection refers to:
- ANSI/AAMI PB70 Level 3 protection or equivalent; or
- ANSI/AAMI PB70 Level 4 protection or equivalent.
Gloves Not Intended for a Medical Purpose
Gloves Not Intended for a Medical Purpose Gloves are devices when they meet the definition of a device set forth in section 201(h) of the FD&C Act. Under section 201(h) of the FD&C Act, these products are devices when they are intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease.
Other gloves are marketed to the general public for general, non-medical purposes, such as use in construction and other industrial applications. Because they are not intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, FDA device marketing authorization is not required, and all the other requirements of the FD&C Act do not apply to manufacturers, importers, and distributors of these products.
Gloves are devices when they are intended for a medical purpose, such as prevention of infectious disease transmission (including uses related to COVID-19). Gloves are not devices when they are intended for a non-medical purpose, such as for use in construction. When evaluating whether these products are intended for a medical purpose, among other considerations, FDA will consider whether:
1) they are labeled or otherwise intended for use by a health care professional;
2) they are labeled or otherwise for use in a health care facility or environment; and
3) they include any drugs, biologics, or anti-microbial/anti-viral agents.
Patient Examination Gloves
A non-powdered patient examination glove is a disposable device intended for a medical purpose that is worn on the examiner’s hand or finger to prevent contamination between patient and examiner. A non-powdered patient examination glove does not incorporate powder for purposes other than manufacturing. The final finished glove includes only residual powder from manufacturing.
These devices are class I (reserved) and subject to premarket notification requirements under section 510(k) of the FD&C Act because general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device and the devices are intended for a use which is of substantial importance in preventing impairment of human health or presents a potential unreasonable risk of illness or injury, under sections 513(a)(1)(A) and 510(l)(1) of the FD&C Act.
FDA currently believes such devices would not create such an undue risk where:
• The product includes labeling that accurately describes the product as an “unpowdered glove” (as opposed to a surgeon’s or patient examination glove), accurately describes its sterility status when individually packaged (non-sterile), does not claim the product as being free of a specific material (e.g., latex free), and includes a list of the body contacting materials;
• The product includes labeling with general statements and makes recommendations that would reduce sufficiently the risk of use, for example, a general statement about devices that have not been cleared by FDA and recommendations against use: when FDA-cleared gloves are available,in surgical settings or where significant exposure to liquid bodily or other hazardous fluids may be expected, and in clinical settings where the infection risk level is high; and
• The product is not intended for any use that would create an undue risk in light of the public health emergency, for example, the labeling does not include uses with chemotherapy drugs, fentanyl, and other opioids, uses for allergy or dermatitis prevention, uses for antimicrobial or antiviral protection, or uses for infection prevention or reduction.
Surgeon’s Gloves
A non-powdered surgeon’s glove is a device intended to be worn on the hands of operating room personnel to protect a surgical wound from contamination. A non-powdered surgeon’s glove does not incorporate powder for purposes other than manufacturing.
The final finished glove includes only residual powder from manufacturing. These devices are class I (reserved) and subject to premarket notification requirements under section 510(k) of the FD&C Act because general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device and the devices are intended for a use which is of substantial importance in preventing impairment of human health or presents a potential unreasonable risk of illness or injury, under sections 513(a)(1)(A) and 510(l)(1) of the FD&C Act.
FDA currently believes such devices would not create such an undue risk where:
- The product meets the standard specification consistent with the consensus standard ASTM D3577: Standard Specification for Rubber Surgical Gloves;
- The product includes labeling that accurately describes the product as being unpowdered, accurately describes its sterility status (sterile) and the sterilization method used, does not claim the product as being free of a specific material (e.g., latex free), and includes a list of the body contacting materials;
- The product includes labeling with general statements and makes recommendations that would reduce sufficiently the risk of use, for example, a general statement about devices that have not been cleared by FDA and recommendations against use when FDA-cleared surgeon’s gloves are available; and • The product is not intended for any use that would create an undue risk in light of the public health emergency, for example, the labeling does not include uses with chemotherapy drugs, fentanyl, and other opioids, uses for allergy or dermatitis prevention, uses for antimicrobial or antiviral protection, or uses for infection prevention or reduction.
Full Guidance: https://www.fda.gov/media/136540/download